Introduction:Thrombocytopenia is a common complication after hematopoietic stem cell transplantation (HSCT) and is strongly associated with transplant-related morbidity and mortality. According to the statistics, the incidence of post-HSCT thrombocytopenia is about 5%-37%. So far, there is no standard treatment regimen for thrombocytopenia after HSCT.
Recombinant human thrombopoietin (rhTPO) can act on megakaryocytes alone and has been wildly used in clinical practice. Thrombopoietin receptor agonists (TPO-RAs), another type of drugs that can increase platelet count, has been available since 2008. It can bind to the TPO receptor and cause the conformational change of the receptor, and then activate the JAK2/STATE5 pathway to increase the proliferation of megakaryocyte progenitors and platelet production.
There have been many prospective or retrospective studies discussing the efficacy and safety of rhTPO and TPO-RAs monotherapy in patients with thrombocytopenia. In clinical practice, it is very meaningful to clarify whether the combination of two types of drugs has a synergistic effect when one type of drug has poor efficacy.
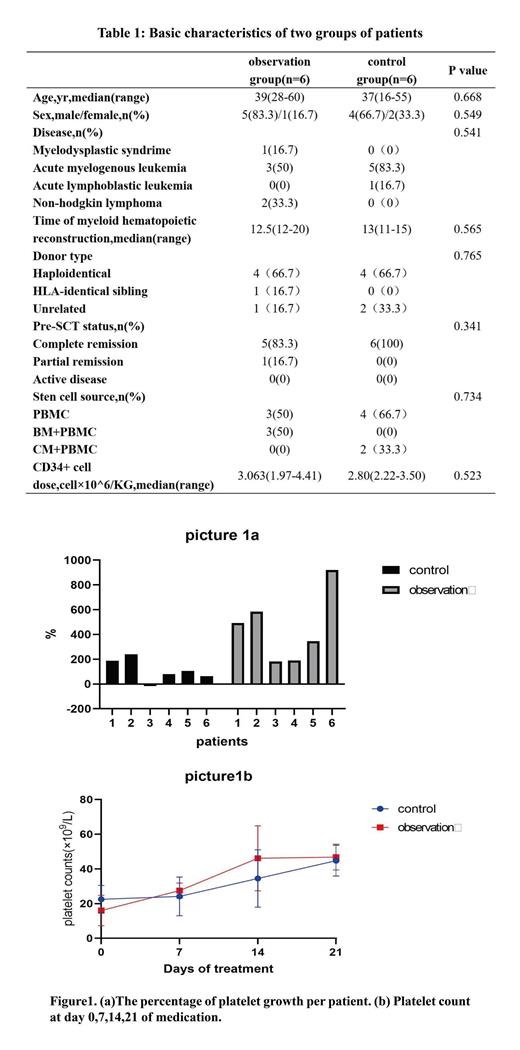
M ethods This study is a retrospective study in total 12 patients from 2022 to 2023. We selected a total of 6 patients who underwent HSCT in our center during the past year, and used rhTPO combined with avatrombopag to help platelet recovery as observation group. Then, 6 patients treated with rhTPO alone with no statistical difference at baseline were selected as the control group. The basic characteristics of these patients are shown in the table1. All the patients were informed and consented to use rhTPO+TPO-RA (avatrombopag) or rhTPO alone. If platelet count <20×10 9/L during treatment,which will be treated with platelet infusion. All the patients were treated with a modified BUCY regimen as the conditioning regimen and ATG+MMF+cyclosporine+short-range MTX were used to prevent graft vs host disease (GVHD). In observation group, the patients were treated with rhTPO 15000U qd i.m and avatrombopag 40mg qd po, while in control group the patients were treated with rhTPO 15000U qd i.m alone. We recorded the platelet counts of the two groups at day0,7,14,21. Based on this data, the percentage increase and growth rate of platelets were calculated. (the percentage increase: difference in platelet count before and after medication/platelet count before medication ×100%; the growth rate: difference in platelet count before and after medication/duration of medication.) The above two values were used to evaluated the efficacy of two treatment protocols. Adverse events occurred during treatment including fever, weakness, liver damage, joint pain, bleeding and thrombosis would be recorded to evaluate the safety.
Results In observation group, patients' platelet counts increased by an average was 54×10 9/L (range 40 to 69). In control group, the average and median increase in their platelet counts was 22 (range -2 to 49). P=0.025 (P<0.05). In terms of the percentage increase in platelet count, the average increase was 452% in observation group VS. 110% in control group. p=0.022(P<0.05). Refer to Figure1a. The average growth rates of platelet counts in observation group were 2.03×10 9/L per day. The averagerate of increase in control group were 1.07×109/L per day. P=0.041(P<0.05). The mean platelet count of the two groups at various time points were shown in Figure1b. None of the patients had any adverse events occurred. No patient developed clinical meaningful abnormality of liver function and discontinued avatrombopag or TPO treatment due to intolerability and adverse effects.
Conclusion Our data shows that the treatment protocol of avatrombopag combined with rhTPO may have a greater effect on platelet growth and boost a faster platelet growth rate. Importantly, no additional adverse effects occurred when the addition of avatrombopag.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal